Research Projects
Tubulin Gene Expression
In eukaryotes, tubulin proteins are encoded by large multi-gene families of alpha- and beta-tubulin subunits. Most cell types express only a subset of the encoded isoforms. Which of the subunits are expressed is determined transcriptionally, but the regulatory networks remain only partially understood.
The level of tubulin mRNAs, however, is controlled through a post-transcriptional mechanism known as tubulin autoregulation. Namely, when in excess of cellular need, mature tubulins negatively regulate the stability of their encoding mRNAs. This process requires a specific recognition of the nascent tubulin proteins as they emerge from the ribosomes. Notably, the first four amino-acids serve as a beacon for mRNA decay. We have recently shown that cells deploy a specificity factor that recognizes this beacon and triggers degradation of mRNA.
Although known for over forty years now, we still don't understand how tubulin autoregulation works or what its physiological importance is. In our work, we deploy biochemical approaches, fluorescent imaging, and a range of genetic and pharmacological tools to address these unsolved problems.
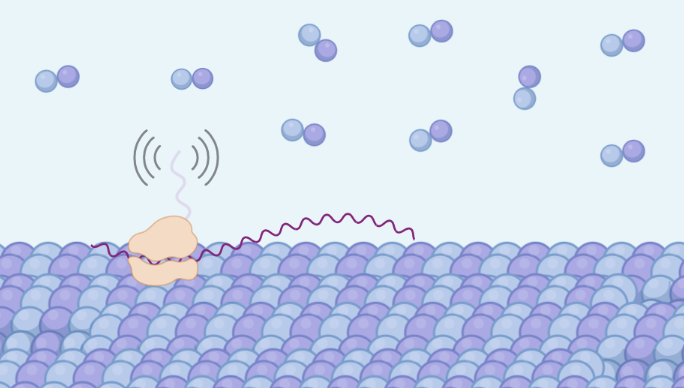 Cotranslational protein quantity control: nascent proteins acting as a beacon for quantity control. Illustration created with Biorender.
Cotranslational protein quantity control: nascent proteins acting as a beacon for quantity control. Illustration created with Biorender.
Tubulin Quantity And Microtubule Homeostasis
In cells, alpha- and beta-tubulins form heterodimers, which further polymerize into microtubules. Microtubules are remarkably dynamic, undergoing phases of growth through the addition and shrinkage through loss of tubulin heterodimers. This behavior is largely influenced by the availability of tubulin heterodimers. In vitro, microtubule dynamics directly depend on the concentration of tubulin subunits. But whether that is true in cells remains unknown.
Precisely regulated microtubule dynamic behaviour is instrumental for microtubules to carry out their numerous cellular functions, such as powering the division of the genetic material, organizing the cytoplasm, or mediating intracellular transport. Our most recent work demonstrates that loss of tubulin autoregulation compromises the function of the microtubule cytoskeleton during cell division. One exciting possibility is that the autoregulation pathway feeds into microtubules' dynamic behavior. We are keen to understand the underlying principles of how loss of tubulin autoregulation compromises mitotic fidelity. To solve this enigma, we combine cutting-edge high-resolution quantitative microscopy and automated image processing with modern chemical biology and pharmacology.
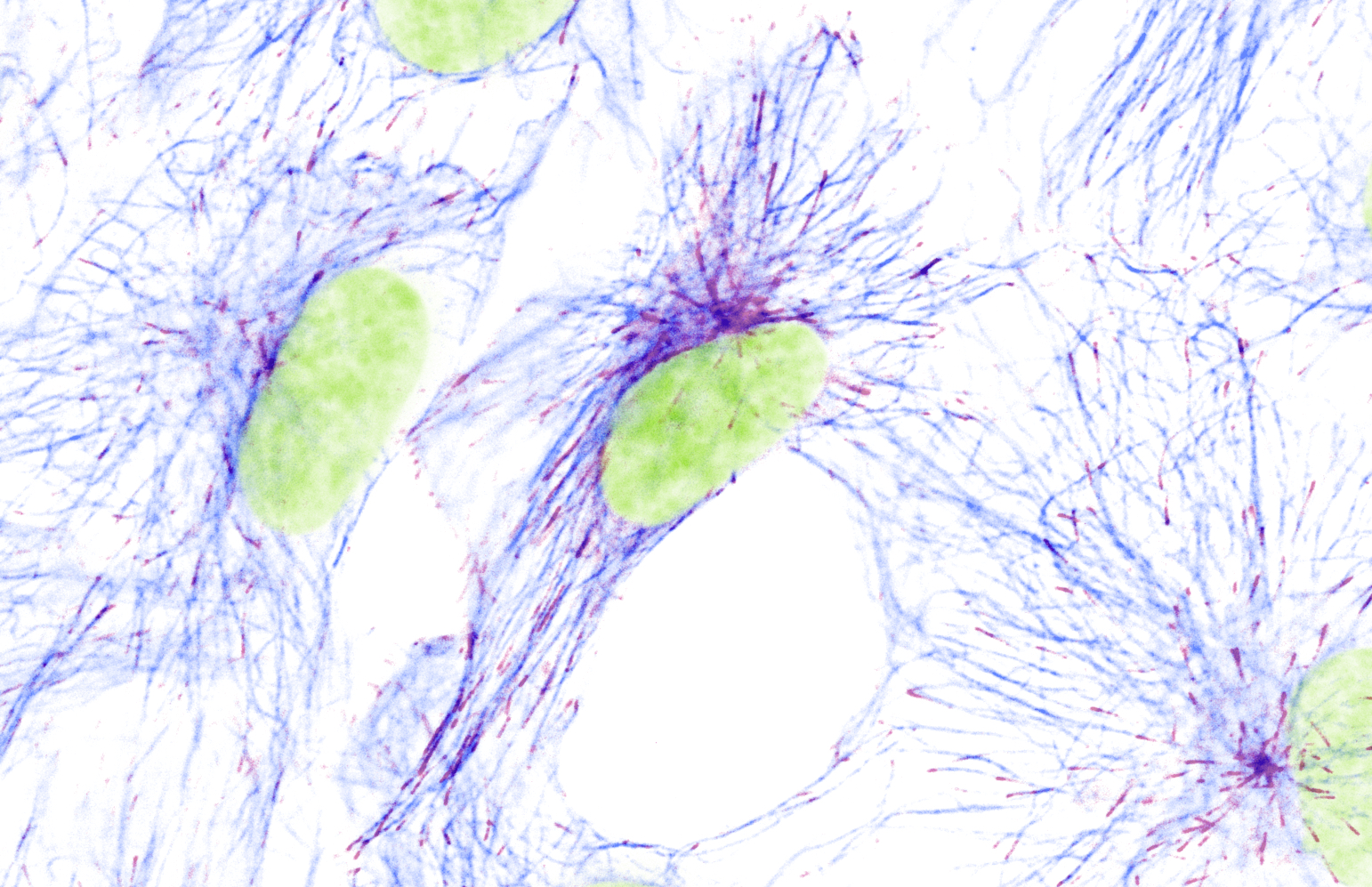 Microtubules (indigo) and their actively growing tips (bordeaux) in mammalian cultured cells. Nuclei are depicted in green.
Microtubules (indigo) and their actively growing tips (bordeaux) in mammalian cultured cells. Nuclei are depicted in green.
Microtubule Nucleation
In vitro tubulin heterodimers can self-polymerize into microtubules in a concentration-dependent manner. But here is a problem: tubulin concentration in cells is well bellow that one required for them to self-polymerize. So how do cells initiate and maintain tubulin polymerization to form microtubules? To circumvent this problem, and we would argue to control it, cells evolved microtubule organizing centres (MTOCs, a.k.a centrosomes in higher eukaryotes, or spindle pole bodies in yeasts). MTOCs are large protein assemblies that concentrate tubulin heterodimers and form a scaffold onto which these are assembled, thus serving as nucleation sites.
The number of MTOCs and their capacity to catalyze tubulin polymerization must be precisely regulated to maintain cellular fitness. Most cell types contain one MTOC which duplicates prior to mitosis such that each daughter cell inherits one MTOC. When things go out of control and supernumerary MTOCs occur, cells undergo devastating divisions that cause massive damage to the genetic material and abnormal karyotypes, which can lead to carcinogenesis or cell death. So how do cells ensure that they have one and only one MTOC that has the right capacity to nucleate microtubules? Our team is using biochemistry, advanced genetic manipulations, and high-resolution microscopy to answer this medically-relevant question.
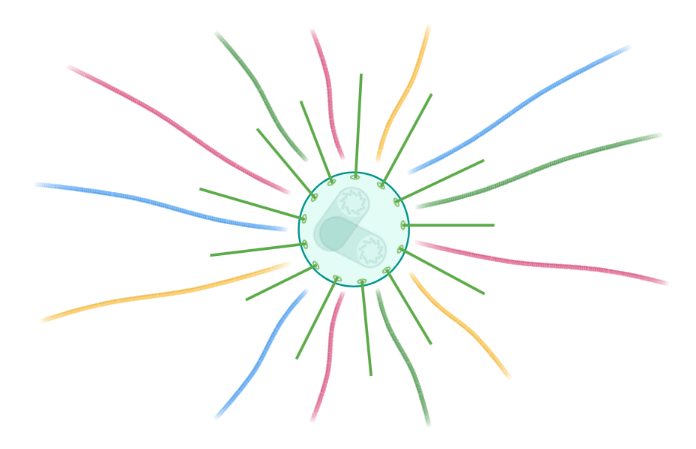
Centrosome-driven microtubule nucleation. Schematic created with Biorender.
