Research Projects
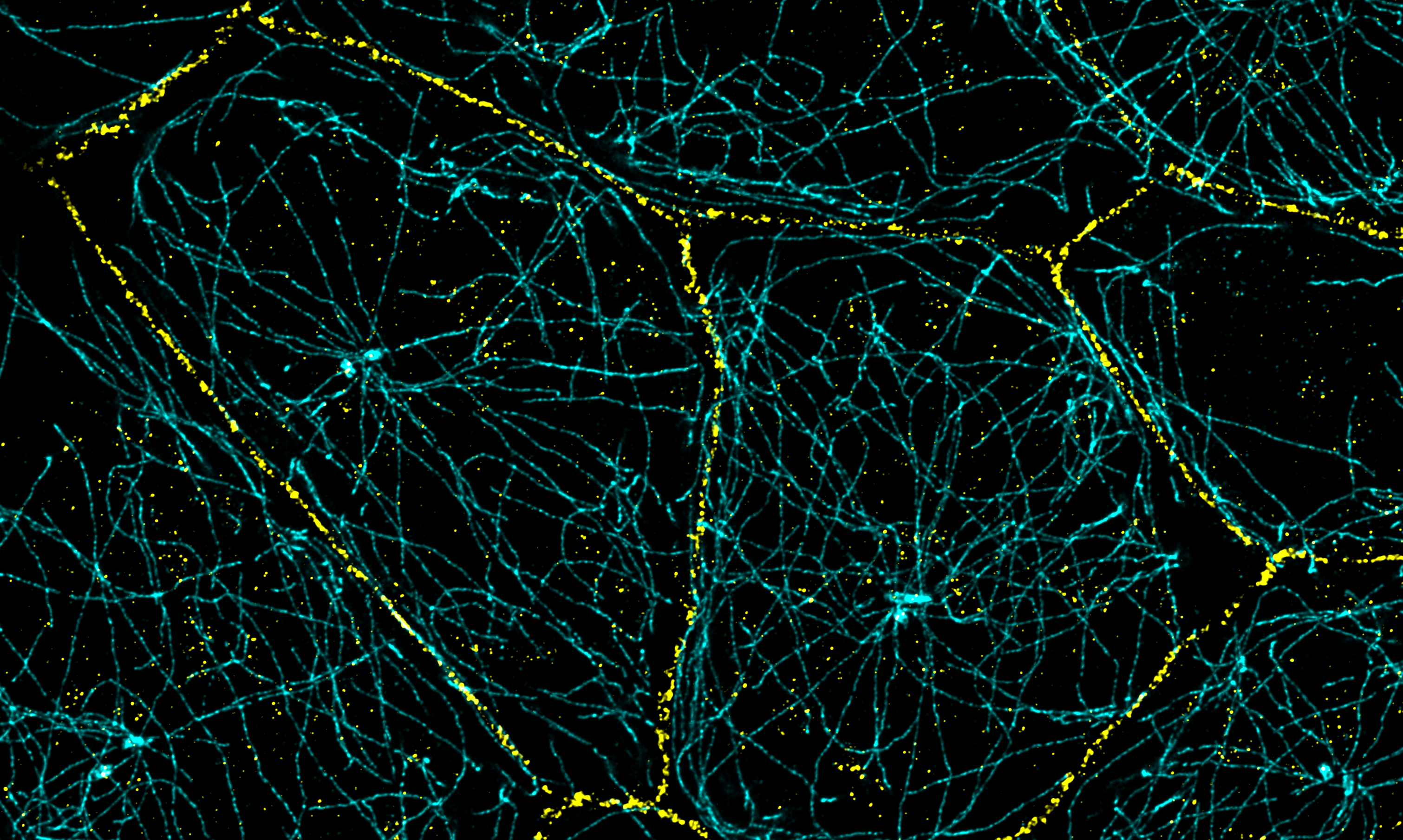
Cell junctions (yellow) and microtubules (cyan) in epithelial cells.
The research projects of the Citi Laboratory address several major scientific questions concerning apical junctions of vertebrate epithelial and endothelial tissues, which play a fundamental role in in separating different body compartments, and in the absorption and secretion functions of major organs. Also, the majority of cancers arises within epithelial organs, invasion and metastasis correlate with disruption or loss of cell-cell junctions, epithelial tissuesare the first target of pathogens, and epithelial cells express on their surface receptors and ion pumps that are fundamental for organism physiology. Since a key feature of epithelial and endothelial tissues is the presence of junctions, understanding the role of junctional proteins in many facets of cellular and organism physiology and pathology is a fundamental question.
The Citi laboratory has focused much of its research on clarifying how specific protein components of epithelial tight and adherens junctions regulate cell architecture, mechanics of the membrane cortex, signaling and different aspects of epithelial cell and tissue function, including barrier, adhesion, pathogen interaction, and ion transport.
Concerning the mechanobiology of the junctional complex, a crucial question is to understand how mechanical force affects the conformation and molecular interactions of specific junctional proteins, and how connections of junctions to the actomyosin cytoskeleton determines the mechanical properties of the plasma membrane cortex. In this context, wediscovered in 2017 that ZO-1 is a mechanosensing protein (Spadaro et al, 2017 Current Biology). In 2022 we discovered that cingulin interaction with ZO-1 regulates ZO-1 conformation (Vasileva et al, JBC 2022), and in 2023 we showed that cingulin and paracingulin tether nonmuscle myosins-2 (NM2s) to junctions to regulates ZO-1 conformation, apical membrane cortex stiffness, tight junction membrane tortuosity and adherens junction organization. The functional connection of cingulin to myosins was first indicated by its original discovery as a protein that copurified with nonmuscle myosin-2 (Citi et al, Nature 1988, Citi et al, JCS 1989), and was confirmed by the demonstration that cingulin binds to NM2 in vitro in a pulldown assay (Cordenonsi et al, JCB 1999). Moreover the cingulin rod sequence shows highest homology to the rod region of NM2s, although the specific coiled-coil sequences of cingulin do not predict filament assembly (Citi et al, J. Struct. Biol 2000). The more precise mapping of the regions of NM2s and cingulin involved in their interaction, and the role of cingulin in recruiting NM2B to tight junctions of MDCK cells in vitro established the details of the functional interaction between cingulin and NM2s in our recent JCB paper (Rouaud et al, JCB 2023). In addition, our 2025 paper (Mauperin et al, Nat. Comm. 2025) showed how gamma-actin, whose TJ proximity is affected by the KO of cingulin, regulates apical membrane cortex stiffness and is part of a mechanochemical feedback circuitry whereby cells respond to the loss of gamma actin by up-regulating NM2A, with downstream mechanical phenotypes. These observations together indicate that cingulin and paracingulin play an important role in organizing the apical actomyosin cytoskeleton and connecting it to tight and adherens junctions to transmit the appropriate force to the membrane cortex.
Cingulin and paracingulin are not only associated with the actomyosin cytoskeleton, but also with microtubules. We showed that paracingulin and not PLEKHA7 anchors the microtubule minus-end binding protein CAMSAP3 to apical junctions, and that depletion of paracingulin both in cultured cells and intestinal cells in mice in vivo results in disordered microtubule organization and altered epithelial apicobasal polarity (Flinois et al JCS 2024).
Concerning signaling, our most recent work highlights a new role of paracingulin in the control of kidney physiology and ionic homeostasis through an effect on angiotensin-2 signaling, which mechanistically explains how the loss of paracingulin prevents development of hypertension in different experimental models in vivo (Rouaud et al, Am. J. Physiol. 2025). Previously, we characterized the role of both cingulin and paracingulin in the regulation of RhoA and Rac1 activities and gene expression in different experimental models in vitro (cultured epithelial cells, embryoid bodies), through their interaction with GEFs and GAPs for Rho GTPases (Guillemot and Citi, MBoC 2006, Guillemot et al MBoC 2008, Guillemot et al MBoC 2014).
We discovered PLEKHA7 as a paracingulin-interacting protein localized at a junctional site distinct from both E-cadherin and ZO-1 (Pulimeno et al, PlosOne 2010, Pulimeno et al JBC 2011), and this discovery was the beginning of a fruitful series of studies on WW-PLEKHA proteins, their interactors and their role in cell and tissue physiology. We discovered PDZD11 as a PLEKHA7-interacting protein and characterized its role in adherens junction assembly (Guerrera et al, JBC 2016), and showed how both proteins are involved, via their interaction with the tetraspanin Tspn33, in the junctional anchoring of the Staphylococcal apha-toxin receptor ADAM10, and consequent toxin-induced cell death (Popov et al, PNAS 2015, Shah et al, Cell Reports 2018, Rouaud et al, JBC 2020). We further discovered PLEKHA5 and PLEKHA6 as PDZD11 interactors, and showed how the three WW-PLEKHA proteins, together with PDZD11, are crucial in the membrane delivery of the copper pump ATP7A to the apical membranes, and for copper homeostasis (Sluysmans et al MBoC 2021, Sluysmans et al Front. Cell Dev Biol 2021). In the same line of studies, concerning the relevance of junctions in physiology and pathology, we also showed that the coronavirus receptor ACE2 is localized at junctions of epithelial cells (Rouaud et al , Cells 2022) and that PLEKHA7-PDZD11 regulates calcium handling by affecting the trafficking and localization of the calcium pump PMCA (Sluysmans et al JBC 2022).
Our interest in the role of junctional proteins in disease, notably cancer, has led us to discover how different lung cancers are distinguishable by the pattern of expression of claudin-1 and claudin-5 (Paschoud et al, Mod. Pathol. 2007), and how PLEKHA7 expression is lost is aggressive breast cancers (Tille et al, PloSOne 2015). Moreover, the observation that histone deacetylase inhibitors up-regulate the expression of tight junction proteins points to their significance in epithelial differentiation (Bordin et al, Mol. Cancer Res 2004).
Our interest in ZO proteins has led us to revise previous models and show that the transcription factor DbpA is controlled by both ZO-1 and ZO-2, and not only by ZO-1 (Spadaro et al JBC 2014). We also mapped the binding site of the original anti-ZO-1 monoclonal antibody R40.76 to the differentially spliced alpha-domain (Rouaud et al, Tissue Barriers 2019) and characterized how cingulin-ZO-1 interaction is a major element in the architecture of the linkage of tight junctions to the actin cytoskeleton (D’Atri et al JBC 2001, Vasileva et al JBC 2022, Rouaud et al JCB 2023). In work carried out at the University of Padova, the Citi laboratory studied tight junctions in Xenopus laevis embryos by immunocytochemical, electron microscopy and biochemical approaches, and described for the first time how cingulin and ZO-1 (this latter in collaboration with the Hausen laboratory) get recruited in distinct manners to the new junctions, and how occludin is a substrate for protein kinase CK2 (Cardellini et al, Dev. Dyn. 1996, Cordenonsi et al, JCS 1997, Cordenonsi et al, Eur. J. Biochem. 1999, Fesenko et al, Development, 2000).
To achieve the results above, we used many different biochemical, in vitro cell biology and in vivo approaches, including the generation and characterization of knockout mice, which have helped us to frame the significance of our discoveries within the context of a whole vertebrate organism. We showed that mice KO for either cingulin or paracingulin are viable, and the KO of cingulin in either embryoid bodies, mice or cultured MDCK cells does not result in altered barrier function in unchallenged situations, although it affects expression of selected genes (Guillemot et al, JCS 2004, Guillemot et al JCS 2012). As noted above, paracingulin-KO mice show altered epithelial polarity, and a blunted response to angiotensin-2 in an experimental model of hypertension (Flinois et al, JCS 2024, Rouaud et al, Am. J. Physiol. 2025). We also generated PLEKHA7-KO mice, which do not show altered epithelial polarity nor altered localization of CAMSAP3 in vivo (Flinois et al, 2024). We also generated and validated several polyclonal and monoclonal antibodies against junctional proteins, that have allowed us and our collaborators to address many biological questions.
The Publications page lists additional publications from the Citi laboratory, or from laboratories with which we collaborated, that are not cited above. We thank all our collaborators for their contribution to our projects, and many colleagues for generous gifts of valuable reagents and materials.
