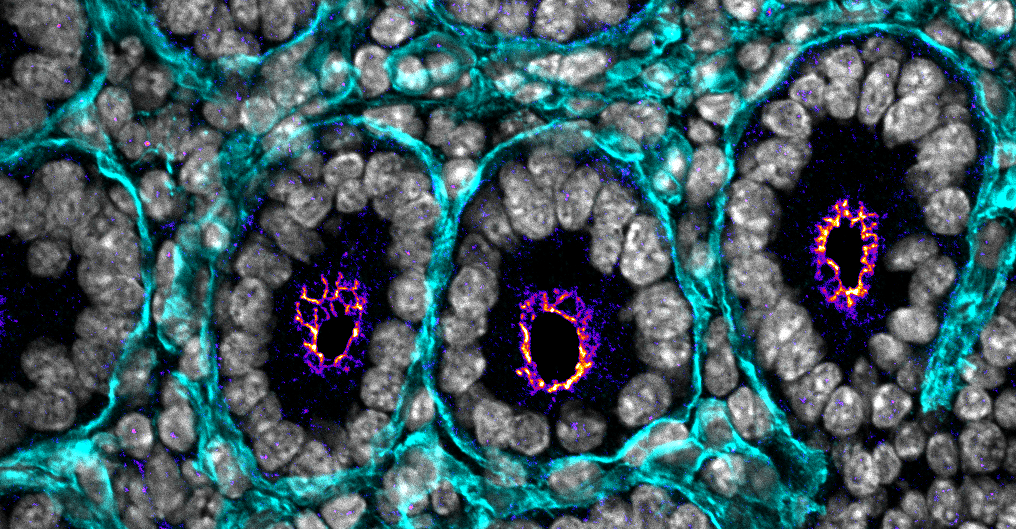
Architecture, mechanics and functions of cell-cell junctions
The goal of research in the Citi Laboratory is to study the role of specific proteins (especially scaffolding and adaptor proteins of tight junctions) and signaling pathways in the architecture, mechanics and functions of apical junctions of epithelial cells in physiology and disease. Our research has revealed role of cingulin and paracingulin in in tethering nonmuscle myosins to junctions to regulate the mechanics of the membrane cortex and in the regulation of Rho GTPases and gene expression in different model systems. The Citi laboratory was the first to identify a role of kinases in integrity of cell-cell junctions and to establish that the major tight junction scaffold protein ZO-1 is a mechanosensing protein. We also discovered PLEKHA7 as a paracingulin-interacting protein and showed that it is localized at junctions at a site disticnt from ZO-1 and E-cadherin, thus identifying a tripartite organization of apical junctions. PDZD11 was characterized as a PLEKHA7-interacting junctional protein and PLEKHA5 and PLEKHA6 as additional PDZD11-interacting proteins. Further work showed how WW-PLEKHA proteins (PLEKHA-5, -6, -7) cooperate with PDZD11 and other partners to control the localization and function of the alpha-toxin receptor ADAM10, the copper transporter ATP7A, and the calcium pump ATP7A. Studies on mice have identified a mechanism through which paracingulin regulates blood pressure, through regulation of kidney ion transporters https://www.rts.ch/audio-podcast/2025/audio/une-proteine-du-rein-impliquee-dans-l-hypertension-28869743.html. For additional information please consult the publication list.
Although retired from the University of Geneva as of Aug. 1, 2025, Prof. Citi is still active in scientific collaborations and is available () for consulting, mentoring and reagents.

 Sandra CITI, MD, PhD
Sandra CITI, MD, PhD