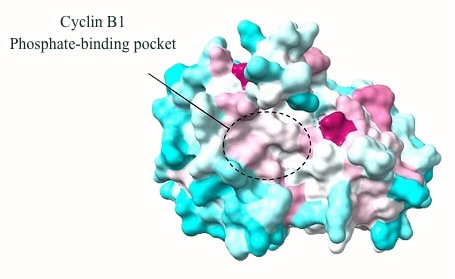
A phosphate-binding pocket in B-type cyclins
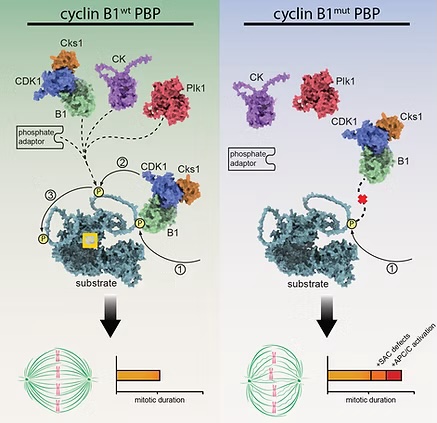
Phosphorylation of substrates by cyclin-dependent kinases (CDKs) is the driving force of cell cycle progression. Several CDK-activating cyclins are involved, yet how they contribute to substrate specificity is still poorly understood. Together with the Thomas Mayer group at the University of Konstanz, we recently discovered that a positively charged pocket in cyclin B1, which is exclusively conserved within B-type cyclins and binds phosphorylated serine- or threonine-residues, is essential for correct execution of mitosis.

HeLa cells expressing pocket mutant cyclin B1 are strongly delayed in anaphase onset due to multiple defects in mitotic spindle function and timely activation of the E3 ligase APC/C. Pocket integrity is essential for APC/C phosphorylation particularly at non-consensus CDK1 sites and full in vitro ubiquitylation activity. Our results support a model in which cyclin B1’s pocket serves as a specificity site factor for sequential substrate phosphorylations involving initial priming events that facilitate subsequent pocket-dependent phosphorylations even at non-consensus CDK1 motifs.
https://www.researchsquare.com/article/rs-4125014/v1